Published
- 2 min read
What is an Electron?
I thought it would be important to cover what exactly an electron is, given that it is the fundamental particle behind much of electromagnetism and, by extension, computing.
Understanding this elementary component is pivotal to a greater comprehension of many mechanics intrinsic to how stuff just works, to be honest.
An electron is one of the basic subatomic particles found within the overall structure of the atom. Its defining characteristic is its negative electrical charge, and it is considered an elementary particle, meaning that it is not comprised of smaller particles, although this is explored further in quantum physics.
The approximate amount of charge 1 electron carries is measured at $-1.602 x 10^-19 Coulombs.
Their mass is approximately 9.109 x 10^-31 kilograms.
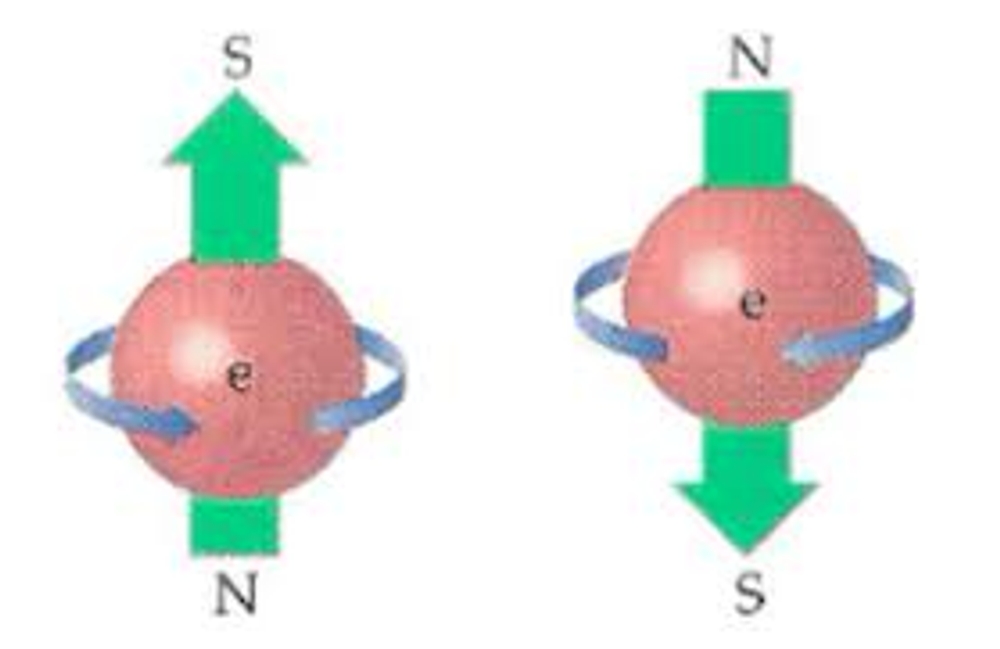
A key property they possess as well is ESR, or Electron Spin Resonance. This is due to the Angular Momentum they posses. However, note that this does not mean that an electron spins in the classical sense. Specifically, when considering this in the context of angular momentum, it simply means that due to changing magnetic fields, it preserves the rotation force applied to it and this can be observed in its magnetic moment. The magnetic moment is simply a measure of the magnetic vector, displayed by its strength and direction, and arises from the aforementioned angular momentum.
In any given axis, an electron is said to be superposed upon—that is, it exists in both a spin-up or spin-down state until observed and measured. This decomposition into a quantifiable state is referred to as waveform collapse. But what is the significance of this property? Well, attraction between two entities in space, electromagnetically speaking, is dictated by paramagnetic and diamagnetic forces. If an atom is paramagnetic, it is said to have unpaired electrons and thus no canceling of its spin, whereas a diamagnetic atom has paired electrons with spins that cancel each other.
The movement of electrons and their behavior as it relates to forming bonds with the atoms of various elements is the key catalyst behind much of Chemistry and by extension a great deal of the physical world.